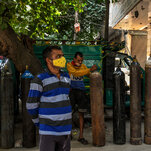CDC and FDA recommend US pause use of Johnson & Johnson’s Covid-19 vaccine over blood clot concerns
The US Centers for Disease Control and Prevention and the US Food and Drug Administration are recommending that the United States pause the use of Johnson & Johnson’s Covid-19 vaccine over six reported US cases of a “rare and severe” type of blood clot.
This is a breaking news story.